PLATFORM
Pharmaceutical Solutions Based on
Long-acting Technology
Platform
이뮤노포지㈜는 반감기 연장 플랫폼 기술 기반의 파이프라인을 보유한 신약개발 회사입니다.
- Home
- >
- Platform
- >
- Platform
ELP Platform
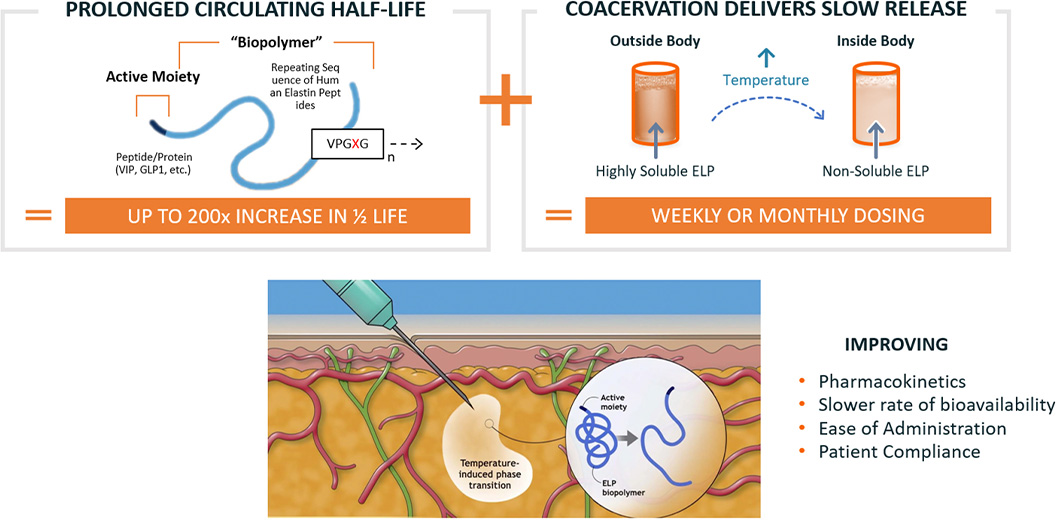
ImmunoForge’s proprietary technology platform for modulating pharmacokinetics has extensive clinical and pre-clinical experience. ELP is based on a naturally-occurring amino acid sequence,
can be optimized for desired drug exposure and has broad utility across a wide-range of drug classes and therapeutic areas. ELP technology extends the circulating half-life of proteins and
peptides and also provides a sustained-release mechanism, resulting in exposure of active molecules for periods of a week or longer from a single subcutaneous injection.
Favorable safety-and-tolerability profile has been well-characterized in multiple clinical trials.
Our proprietary technology platform is based on recombinant biopolymers called elastin-like polypeptides (ELPs), which comprise individual building blocks derived from a five-amino acid
repeat motif found in the human protein elastin. This five-amino acid motif is repeated multiple times to form the ELP biopolymer.
Our ELP technology extends the circulating half-life of proteins and peptides and also provides a sustained-release mechanism, resulting in exposure of active molecules for periods of a week or
longer from a single subcutaneous injection.
Our strategy is to apply our ELP technology to proteins and peptides with well-characterized therapeutic activities but suboptimal half-lives to improve their pharmacokinetics,
enable their use as pharmaceutical products and allow for more convenient dosing regimens.
ELP fusion proteins can undergo a temperature-dependent reversible phase transition. At lower temperatures ELP fusion proteins are completely soluble,
while at warmer temperatures the ELP fusion proteins are in a gel-like state. This allows the ELP fusion proteins to be easily handled and administered subcutaneously using standard,
fine-gauge needles and syringes. Once the ELP fusion protein is exposed to body heat, it forms a drug depot that slowly releases soluble ELP fusion proteins into the circulation.
By modifying the amino acid sequence of the individual subunits and by varying the overall length of the ELP biopolymer, we can engineer our ELP fusion proteins to be released
on timescales extending to a week or longer.
We produce our ELP-based products by engineering E. coli to produce a single protein comprising the active peptide or protein fused to the ELP biopolymer.
This molecule is active as a fusion protein and does not require cleavage or release of the peptide. ELP fusion proteins are produced in the soluble fraction of
E. coli, which allows for ease of scale up and purification.
